X-ray crystallographic studies of a series of penicillin-derived asymmetric inhibitors of HIV-1 protease.
Jhoti, H., Singh, O.M., Weir, M.P., Cooke, R., Murray-Rust, P., Wonacott, A.(1994) Biochemistry 33: 8417-8427
- PubMed: 8031777
- DOI: https://doi.org/10.1021/bi00194a005
- Primary Citation of Related Structures:
1HTE, 1HTF, 1HTG - PubMed Abstract:
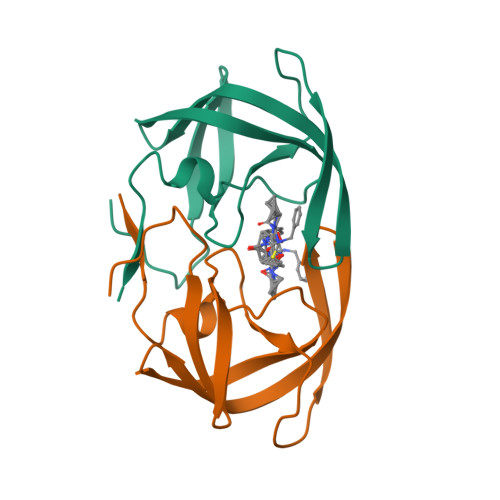
In the development of a treatment for AIDS, the HIV-1 protease has been identified as a good target enzyme for inhibitor design. We previously reported a series of dimeric penicillin-derived C2-symmetric HIV-1 protease inhibitors [Humber, D., et al. (1993) J. Med. Chem. 36, 3120-3128]. In an attempt to reduce the size and optimize the binding of these C2-symmetric inhibitors, molecular modeling studies led to a novel series of monomeric penicillin-derived inhibitors of HIV-1 protease. The binding modes of these monomeric inhibitors have been characterized by X-ray crystallographic and NMR studies. Crystal structures of HIV-1 protease complexed to three inhibitors (GR123976, GR126045, and GR137615) from this series identify the molecular details of the interactions. The binding of GR123976 (IC50 = 2.3 microM) exhibits good hydrophobic contacts but few electrostatic interactions. A strategy of structure-based design and chemical synthesis led to the elaboration of GR123976 to optimize interactions with the protein. Crystallographic analysis of HIV-1 protease complexed to GR126045 and GR137615 identified these interactions with the catalytic aspartates and the protein binding pockets. The crystal structures of the three complexes confirm the presence of the major interactions modeled in order to optimize potency and reveal details of the molecular recognition by HIV-1 protease of this novel series of nonpeptidic inhibitors.
Organizational Affiliation:
Department of Biomolecular Structure, Glaxo Research & Development Limited, Greenford, Middlesex, England.